Global Quality
A global team of professionals proficient in GLP, GCP, cGMP, and CSV standards, with expertise in GxP-compliant quality systems.
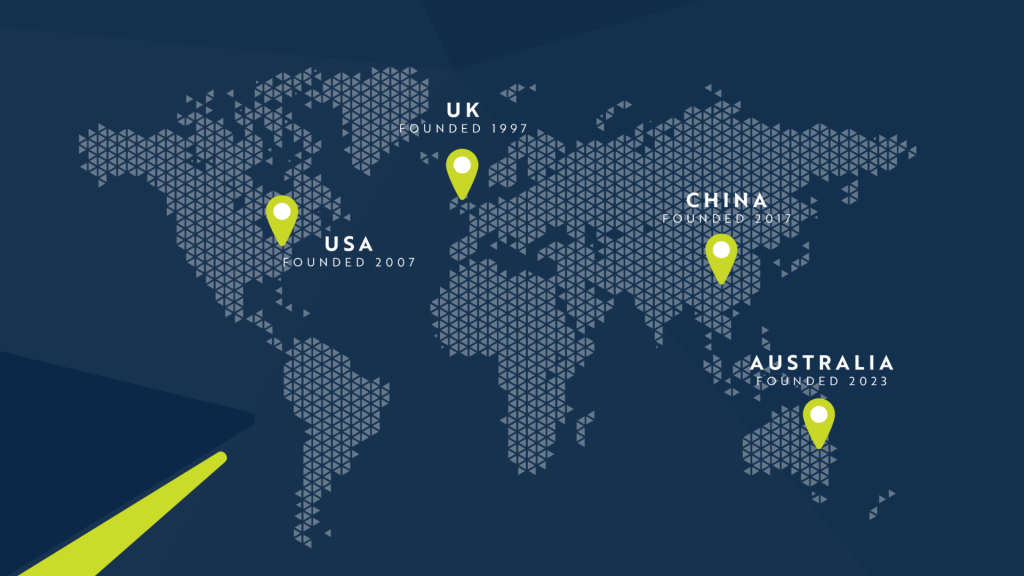
We are proud to house five integrated state-of-the-art laboratories across four continents, all aligned and harmonized through our Global Quality leadership team to ensure the highest standards and all pertinent regulatory requirements are consistently met.
Our team of more than 30 experienced Quality professionals hold many industry accreditations and Quality association memberships. Resolian team leaders regularly present and attend focused conferences and classes to ensure continuous awareness and compliance to our evolving industry.
Reach out to us directly to learn more about Resolian’s Global Quality management services.
Regulatory Compliance and Industry Standards
- GxP implementation and adherence since 1998
- Successful completion of regulatory inspections by the FDA, EPA, EMA, MHRA & NATA
- Breadth of knowledge across GLP, GCP, cGMP and CSV
- Licensed for controlled drugs, radioactivity, animal vivarium, Human Tissue (UK)
- Hosting more than 80 client and regulatory audits per year
- e-document control, audit management and CAPA systems in place
- Risk-based approaches utilized for audits and system validation
Harmonized SOPs and Policies
- Harmonizing core critical SOPs across all Resolian laboratories
- Implemented Global Policies – Data Integrity, Data Privacy, ESG
- Rapid method transfer between Resolian laboratories
- Consistency in processes, methods, and presentations (both regulatory and scientific)
- Establishment of best practices at all locations, drawing on global expertise
- Flexibility to transfer resource and information between laboratories
Our Labs – Global Quality
America

Kingdom






Choose a location
United Kingdom
Bioanalytics Fordham
Your experts in:
- LC-MS
- Immunoassay
- Biomarkers
- Protein and oligonucleotide LC-MS
- Immunogenicity
- Flow cytometry
+ Over 25 years of experience in Europe
Learn MoreAnalytical Sciences Fordham and Sandwich
Your specialist in:
- GMP and CMC testing
- Materials Science
+ Over 20 years of experience in Europe
See Fordham LabSee Sandwich Lab
North America
Bioanalytics Malvern
Your experts in:
- MetID, animal dosing (nonGLP, rodent)
- LC-MS
- Immunoassay
- Protein and oligonucleotide LC-MS
- Immunogenicity
- Biomarkers
- DMPK
- PCR
+ Over 15 years of experience in the US
Learn MoreAustralia
Bioanalytics Brisbane
Your experts in:
- LC-MS
- Immunoassay
- Biomarkers
- Early phase clinical research/phase I studies
Specialists in Australia
Learn MoreChina
Bioanalytics Chongqing
Your experts in:
- LC-MS
- Immunoassay
- Biomarkers
- Protein and oligonucleotide LC-MS
- Antibody Drug Conjugates
- mRNA assays
+ Over 20 years of industry experience
Learn More