Supporting Sciences
Explore the comprehensive supporting CRO services provided by Resolian.
Resolian’s dedicated specialists are committed to delivering a wide range of CRO services, either as standalone solutions or as part of a complete drug development package.
Reach out to us directly to learn more about Resolian’s comprehensive supporting CRO services.
Our Supporting Sciences
Statistical PK Analysis
Offering full bioanalytical and PK/TK support for all stages of drug development.
Global Quality
A global team of professionals proficient in GLP, GCP, cGMP, and CSV standards, with expertise in GxP compliant quality systems.
Other Supporting Sciences
Flow Cytometry Sample Processing Packages
The Flow Cytometry team offers sample processing packages with the aim of stabilizing the sample prior to shipping and decreasing variability.

Logistics and Clinical Study Support
Our bioanalysis, logistics, and sample management teams work together to provide an integrated service for global, multi-center clinical studies for our clients.

Data Management
Delivering comprehensive Data Management services using efficient and meticulous processes, coupled with state-of-the-art technologies to ensure your clinical trial data is managed with precision and accuracy.

Microsampling
Delivering specialized methods and workflows that leverage innovative microsampling technology at the pre-clinical, clinical, and bioanalytical levels.

Document Writing and Publishing
Providing high-quality reports following eCTD guidelines that are customizable to the sponsor’s needs.

Project Management
Our Project Management team has extensive expertise in a wide range of study types and therapeutic areas, and will oversee every aspect of your study.

Statistics
Delivering you timely, high-quality statistical services, in accordance with regulatory requirements.

Sample Management
Our sample management kits provide all the materials necessary for accurate, efficient, and safe preparation, collection, and shipment of clinical samples from trial sites to bioanalytical laboratories.

Archiving
Offering GLP-compliant archiving solutions within an HVAC-controlled environment.

UK Lab Tour
Immunoassay Pharmacokinetics

Pharmacokinetics – Area Under the Curve Calculations
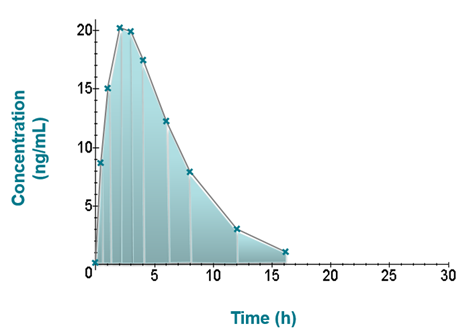
Introduction to Pharmacokinetics

Pharmacokinetics (PK) Urine Analysis

Statistical PK Analysis

Global Quality

Logistics and Clinical Study Support

Data Management

Microsampling
