Home » Our Research
Our Research
Discover our recent findings, catch up with news, and review in-depth resources.

Resolian Acquires China-Based Bioanalytical CRO Denali Medpharma
Resolian, a global bioanalytical contract research organization (CRO) specializing in drug metabolism and pharmacokinetics for small and large molecules, has acquired Denali Medpharma…

How to Build a Comprehensive Response to Client Needs as a CRO
As a CRO, meeting client expectations can be an ongoing challenge. What can organizations do to make sure their research is as effective and efficient as possible?
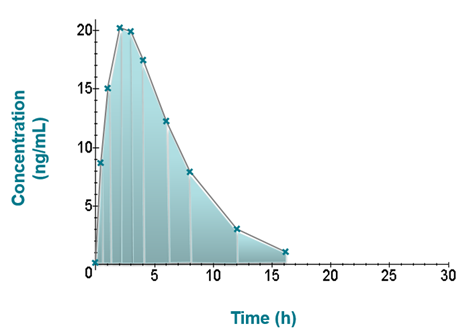
Pharmacokinetics – Area Under the Curve Calculations
The area under the concentration vs. time curve is a useful description of exposure of drug following administration. There are different ways in which AUC may be calculated…

Introduction to Pharmacokinetics
An understanding of the characteristics of drug exposure following administration and how it relates to safety and efficacy is critical for progressing drug development (e.g. in decision making for drug candidates…

The Role of OGNTs in the Personalized Medicine Revolution
This paper highlights oligonucleotides (OGNTs) as a revolutionary new class of drugs in personalized medicine due to their targeted approach to genetic diseases.
Single-Day Protein LC-MS Bioanalysis
This paper explains how next-generation trypsins offer faster protein digestion for LC-MS analysis with comparable performance to standard methods.