Home » Our Research
Our Research
Discover our recent findings, catch up with news, and review in-depth resources.
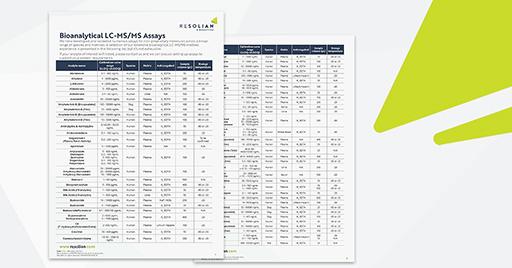
Bioanalytical LC-MS/MS Assay List
With 20+ years of working to GLP and GCP, we have developed an extensive number of LC-MS/MS bioanalytical methods in a wide range of species and matrices.

Immunoassay Pharmacokinetics
Resolian can support all your immunoassay bioanalytical requirements for toxicokinetics (TK) & pharmacokinetics (PK) data collection and interpretation…

Materials Characterization Testing Services
A detailed list of our Materials Characterization testing services.
Why Outsource Foreign Matter Analysis?
It is important to have a good understanding of your manufacturing process in order to implement effective controls to prevent future contamination…

Dive Into the World of Resolian With Our Video Series Featuring Patrick Bennett
Explore the history that shaped Resolian and get a sneak peek into our future plans with our new video series…

Alliance Pharma and Drug Development Solutions Are Now Resolian
Alliance Pharma, a global leader providing bioanalytical, DMPK, and CMC testing services in the pharmaceutical and biopharmaceutical industry, and Drug Development Solutions, Ltd. (acquired in 2022), today announced…