Leading the Way in Secure, Cross-Border Analytical Solutions
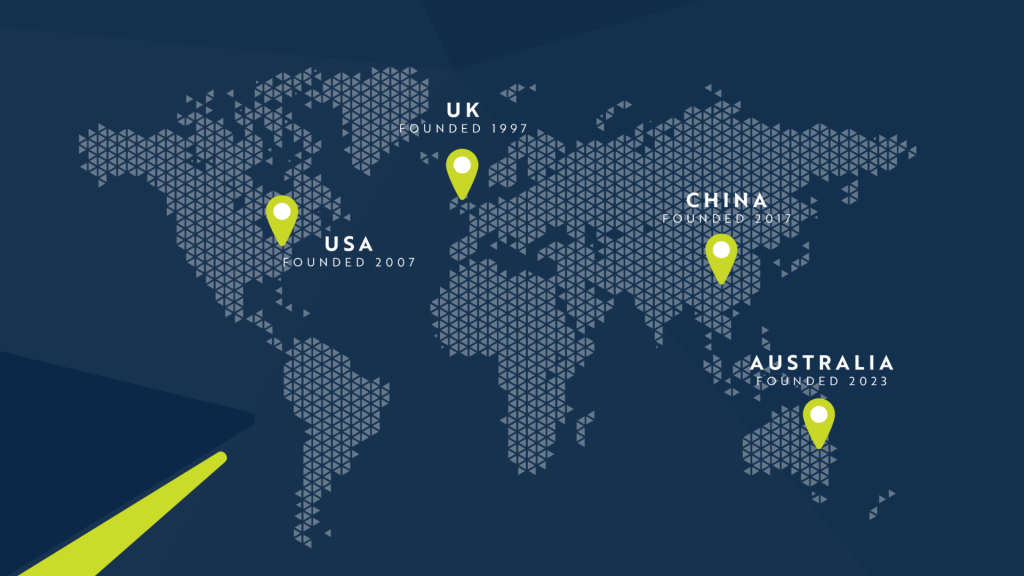
At Resolian, our vision has always been about setting the global standard in laboratory solutions. With five labs worldwide, including our US-owned facility in China, we deliver secure, harmonized services designed to support your success across borders.
Our Chongqing lab operates on a western infrastructure, ensuring all client data is managed with stringent US and European frameworks. This means you get the reliability and security you expect, wherever you are in the world.
We’re committed to delivering seamless scientific expertise that’s globally integrated and locally specialized.
Global Labs. Singular Framework

Min Meng
Chief Operations Officer.
President of Resolian APAC
I have worked for 25+ years in US CROs.
When we set up the China lab, we applied the same western operational framework to ensure seamless integration and top-tier service delivery for global clients.
Quality is Our Priority.
Across Our Global Labs.
Our Chongqing lab is fully integrated with Resolian’s global quality framework, ensuring adherence to the highest international standards. Alongside meeting international GLP and GCP requirements the lab is also ISO 9001 certified, following the same rigorous quality processes as all our sites, ensuring compliance with both Chinese regulatory agencies and global bodies like the FDA.

David Butler
Vice President
Corporate Quality
Proven Expertise. Globally Accredited.
Our Chongqing lab holds global accreditations, meeting the highest regulatory standards, including US FDA compliance and China regulatory approvals, providing you with peace of mind wherever your projects take you.
- FDA Regulatory Assessment Report
- USFCR Verified Vendor
- Successful FDA audit
- Over 25 successful NMPA audits

Secure Health Data.
Protected at Every Step.

Alex Paiva
Senior Director
Global Information Technology
Security is the foundation of our Global IT infrastructure. In Chongqing, our dedicated IT team works closely with Resolian’s global team to ensure secure, compliant management of sensitive health data, utilizing the latest in data protection technologies.
Your Goals. Our Science.
Our lab in Chongqing offers a comprehensive range of services, designed to support your research and development goals, including:
- GxP and non-regulated bioanalysis by LC-MS and Immunoassay
- Biomarkers
- Protein and oligonucleotide LC-MS
- Antibody Drug Conjugates
- mRNA assays
