Small Molecule LC-MS/MS
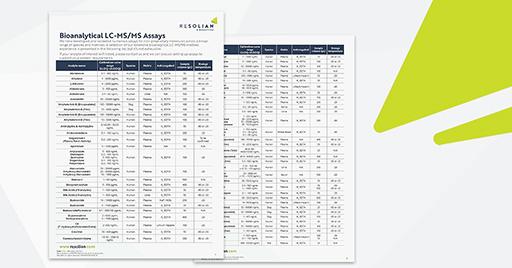
Global capabilities for small molecule LC-MS/MS assays.
Resolian offers key collaborations in GxP clinical and pre-clinical bioanalysis with additional full Discovery Support.
You and your team have direct access to trusted scientists and leaders across our geographies for bioanalysis of small molecular drugs using high-sensitive LC-MS/MS and ICP-MS platforms.
Projects initiate via either de novo method development or technical transfer from your scientists, followed by implementation with validation and sample analysis in compliance with regulatory guidelines to support all stages of pharmaceutical development.
Reach out to us directly to learn more about Resolian’s Small Molecule LC-MS/MS services.
Clinical and IND Enabling Support
- Method development
- Method transfer and optimization
- GxP method validation per FDA/EMA/MHRA/ICH guidance
- Cross-validation within Resolian and external partners
- GLP sample analysis to support pre-clinical TK/PK studies
- GCP standard sample analysis to support all phases of clinical trials
- Clinical sample analysis to support bioequivalence studies

Discovery Support
- Rapid method development of fit-for-purpose methods
- Customized method qualification
- High throughput analysis to support discovery TK/PK/PD studies and in vitro assays
- Homogenization and analysis of tissues to assess drug distribution
- Biomarker analysis for pre-clinical pharmacology studies

Our Bioanalytics Resources
Our Labs – Small Molecule LC-MS/MS
America

Kingdom






Choose a location
United Kingdom
Bioanalytics Fordham
Your experts in:
- LC-MS
- Immunoassay
- Biomarkers
- Protein and oligonucleotide LC-MS
- Immunogenicity
- Flow cytometry
+ Over 25 years of experience in Europe
Learn MoreAnalytical Sciences Fordham and Sandwich
Your specialist in:
- GMP and CMC testing
- Materials Science
+ Over 20 years of experience in Europe
See Fordham LabSee Sandwich Lab
North America
Bioanalytics Malvern
Your experts in:
- MetID, animal dosing (nonGLP, rodent)
- LC-MS
- Immunoassay
- Protein and oligonucleotide LC-MS
- Immunogenicity
- Biomarkers
- DMPK
- PCR
+ Over 15 years of experience in the US
Learn MoreAustralia
Bioanalytics Brisbane
Your experts in:
- LC-MS
- Immunoassay
- Biomarkers
- Early phase clinical research/phase I studies
Specialists in Australia
Learn MoreChina
Bioanalytics Chongqing
Your experts in:
- LC-MS
- Immunoassay
- Biomarkers
- Protein and oligonucleotide LC-MS
- Antibody Drug Conjugates
- mRNA assays
+ Over 20 years of industry experience
Learn More