Peptide Analysis
Experts in offering you bioanalytical solutions using LC-MS/MS technology.
Resolian’s industry-leading bioanalytical solutions have helped clients develop new peptide therapeutics to improve the quality of life.
Reach out to us directly to learn more about Resolian’s Peptide Analysis services.
LC-MS/MS Bioanalysis of Peptides
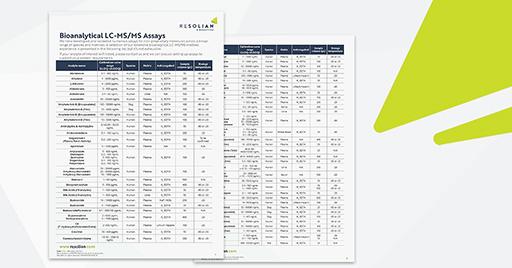
High sensitivity methods are often required for peptide bioanalysis owing to their enhanced efficacy. Resolian’s team has developed and validated methods using LC-MS/MS to quantify levels of peptides in plasma at levels as low as 10 pg/mL.
Often the amino acid composition within peptides or their specific conjugation to other modalities can lead to undesirable binding with labware during extraction or LC-MS/MS measurement. Resolian’s team is experienced in rapidly predicting/diagnosing these issues and implementing solutions to deliver robust methodologies.
Peptides can often be susceptible to degradation, due to the presence of a wide range of endogenous peptidases. Resolian possesses experience in assessing their stability and can advise you on pre-analytical stabilization techniques to control this.
Building upon this, Resolian can implement capabilities in High Resolution MS to identify these degradation products (metabolites) and develop methods to quantify them.
Beyond their experience with peptide bioanalysis, Resolian’s dedicated LC-MS team offers you integrated support from early discovery to late-phase clinical trials, advising you on the regulations applicable to your project.
Integrated Services for Peptides
- Fast method development for discovery compounds
- Method development and validation for GLP TK studies and GCP clinical trials
- Fast turnaround for phase I clinical studies
- Pharmacokinetic data analysis using validated software systems (e.g., WinNonlin)
- Logistical support for clinical sample management including sample collection kit production and supply of sampling kits, labels, investigator manual and shipping
- Data management using LIMS or specialist software