Cell and Gene Therapy
Comprehensive, purpose-built CGT services (including PCR, Flow Cytometry, ELISpot, ADA/nAb, and Biomarkers) to support your research.
Cell and Gene Therapy (CGT) programs demand specific expertise, start-to-finish collaborator engagement and experience with regulators. Resolian’s leaders have 15+ years supporting CGT studies, including contributions to more than 5 US FDA therapeutic approvals.
Resolian can contribute to the overall success of your CGT programs at any stage, from pre-discovery/discovery to clinical trial testing.
Resolian continues to build comprehensive capabilities, fostering a collaborative partnership where the team becomes an integral extension of your research efforts.
Reach out to us directly to learn more about Resolian’s Cell and Gene Therapy services.
Services in Detail
Comprehensive Program Management:
Streamlined sample logistics and services purpose-built for your science and development program design for efficient study execution.
Multimodal Expertise:
Proficiency with CAR-T, AAV, lentivirus, CRISPR/Cas-9, oligonucleotides, plasmid-LNP, mRNA, siRNA, vaccines, plasmids, expressed transgene products, anti-PEG.
Scientific Support Across Disciplines:
Multidisciplinary SMEs across biomarkers, immunogenicity, PD, PK, biodistribution, viral shedding assessments.
Integrated Techniques:
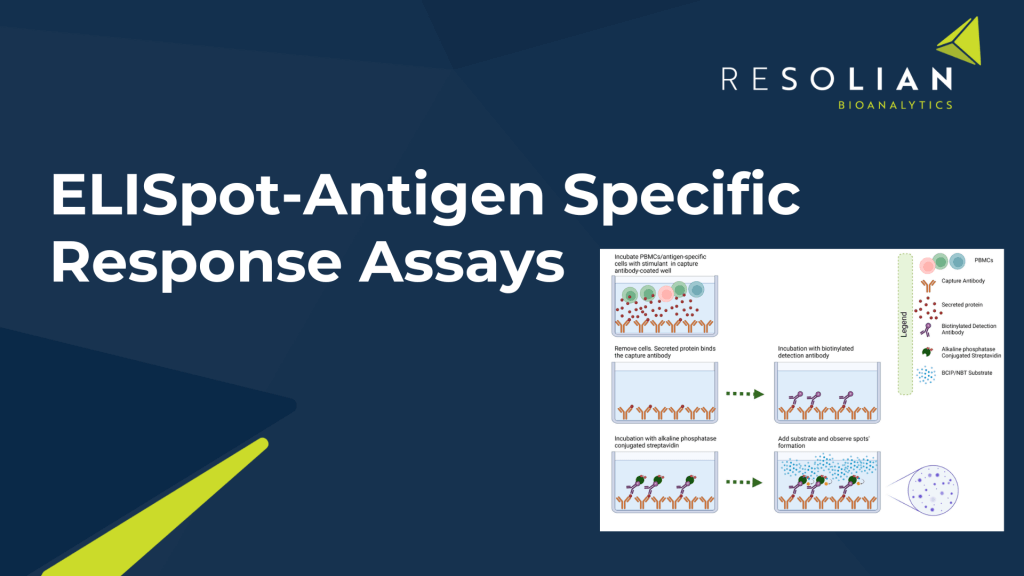
Agile implementation across Flow Cytometry, LC/MS, Immunoassay, ELISpot, and PCR for a holistic approach.
Therapeutic Specialization:
Offering you expertise in rare disease, pediatrics, neurology, cardiovascular disease, immunology, and more, tailoring support to the unique requirements of each indication.
Regulatory Navigation: Active engagement with regulatory bodies, aligning with guidelines and facilitating regulatory approval.