LC-MS/MS assay development within Bioanalysis is driven by developing accurate, precise and robust methods within the shortest time frame possible.
Within our department we have employed a generic protocol to enable a consistent and efficient process to reach these goals.
LC-MS/MS Conditions
Initial Screening
Determine: Log D and pKa to assist in initial decision making
Infusion of analyte into MS
- Pos/Neg, ESI/APCI
- M+H or M-H or Adducts
- Minimise adducts or maximise
Scouting HPLC Gradients
- Acidic & Basic & buffered mobile phases for retention and signal:noise (S:N)
- Gradient for sharper peaks and sensitivity
- Isocratic for resolution
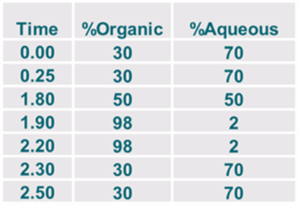
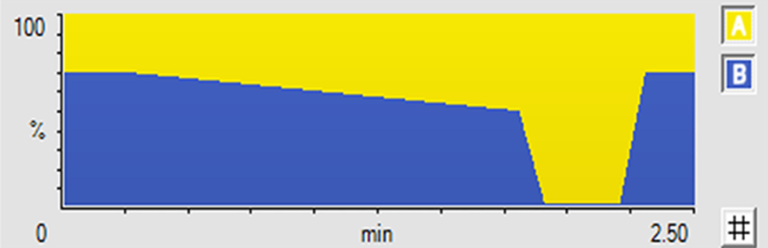
Limit of Quantitation
- Dilution series made in solution
- Provides an idea of what sort of sample extraction technique is needed
- S:N of 20:1 to enable an ↑chance it will be suitable in matrix
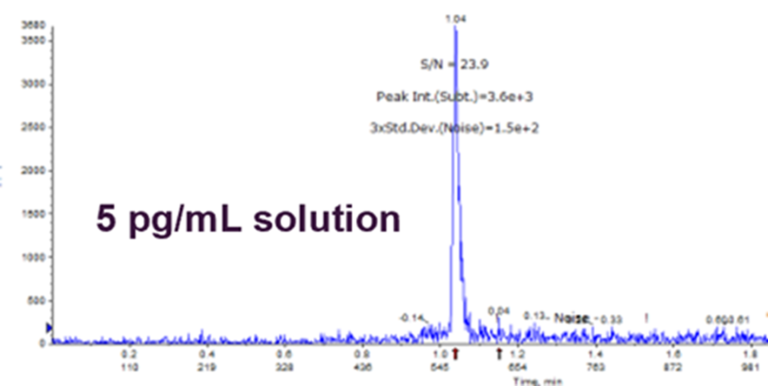
Carryover
If observed will need to locate the source:
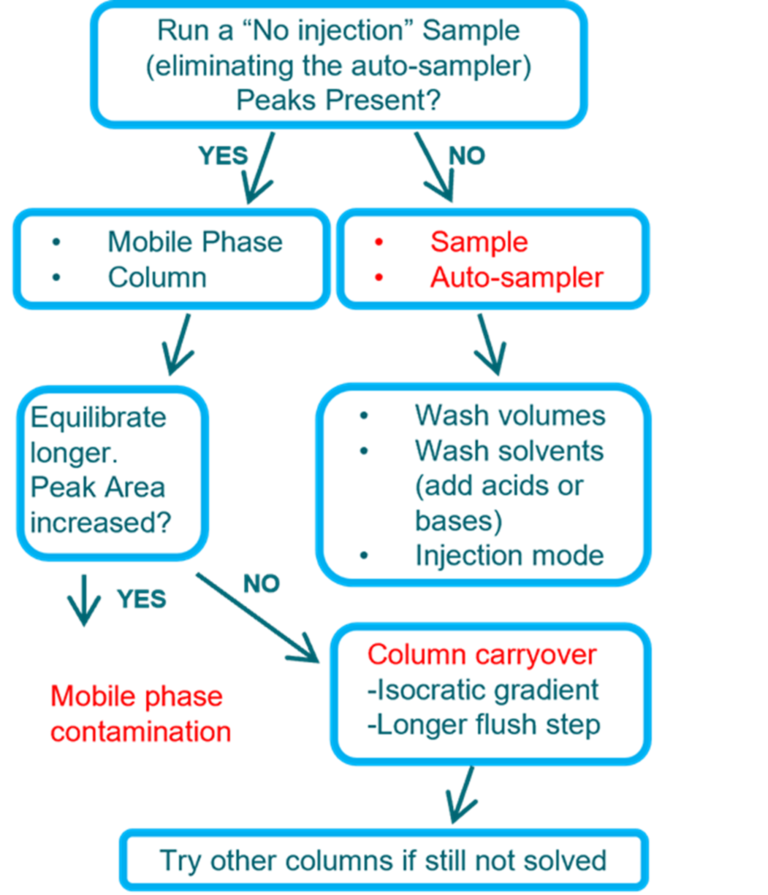
Sample Extraction
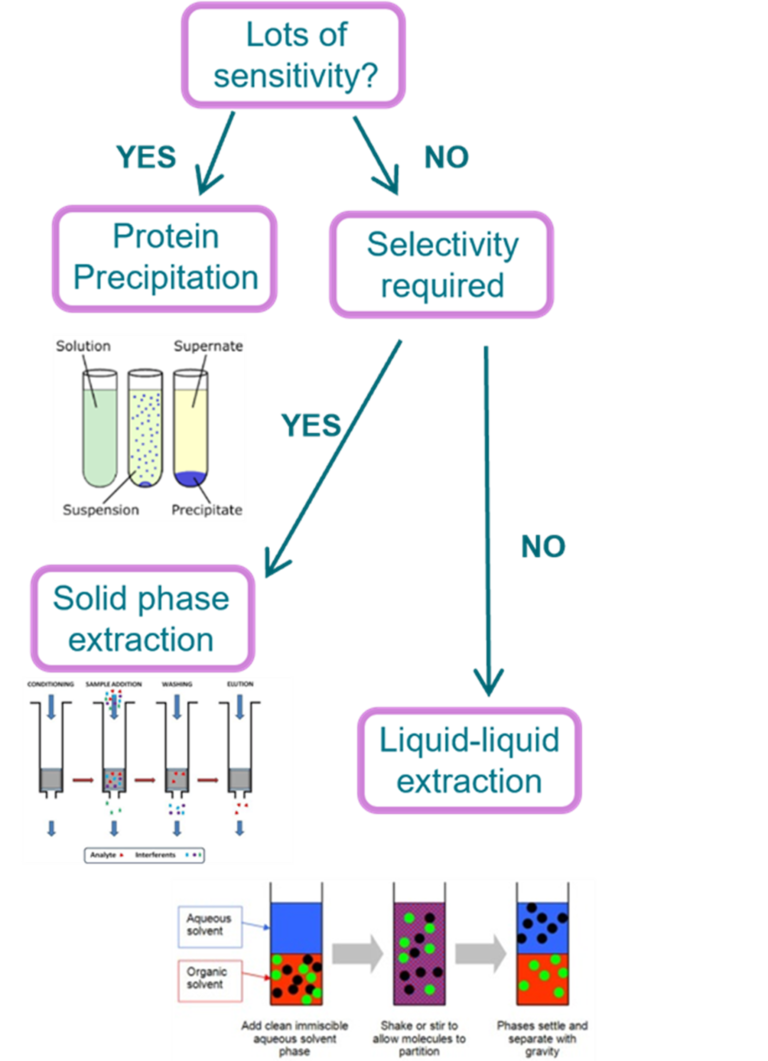
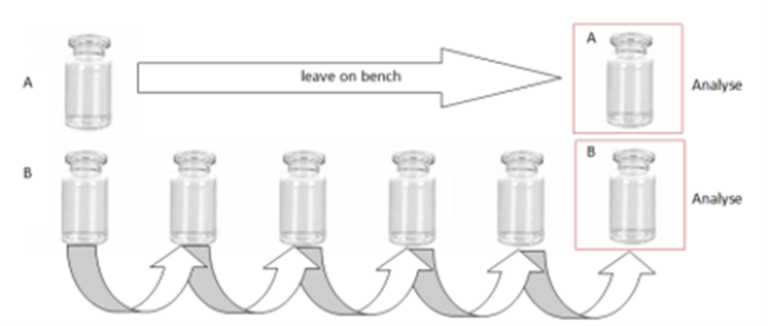
Binding
Non-specific Binding
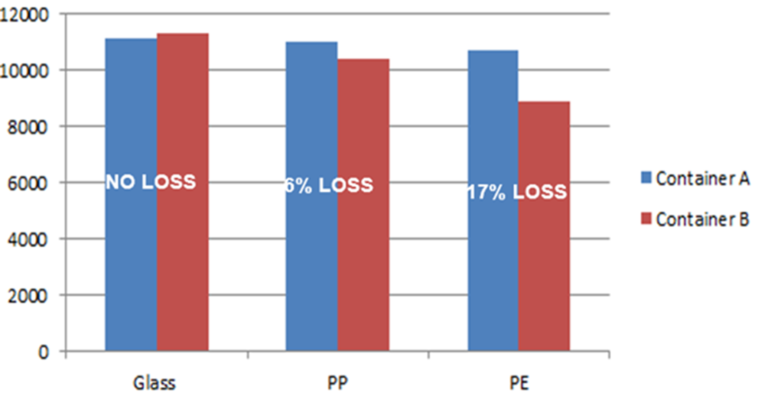
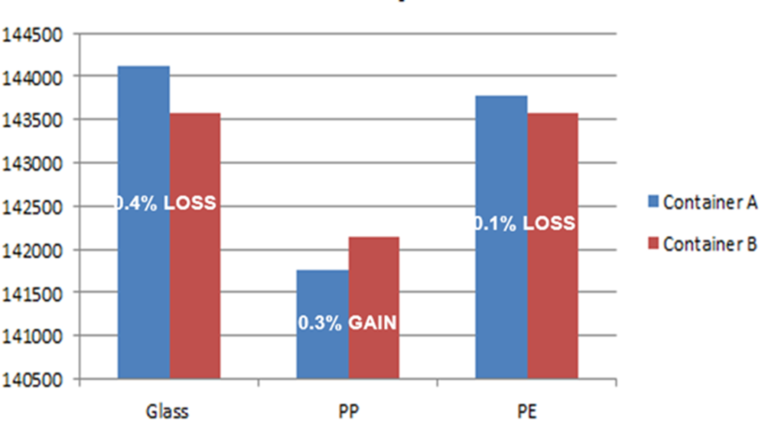
Binding test performed as follows in 3 types of materials:
- Glass
- Polypropylene
- Polyethylene
Analyte 1
Analyte 2
Binding in Urine
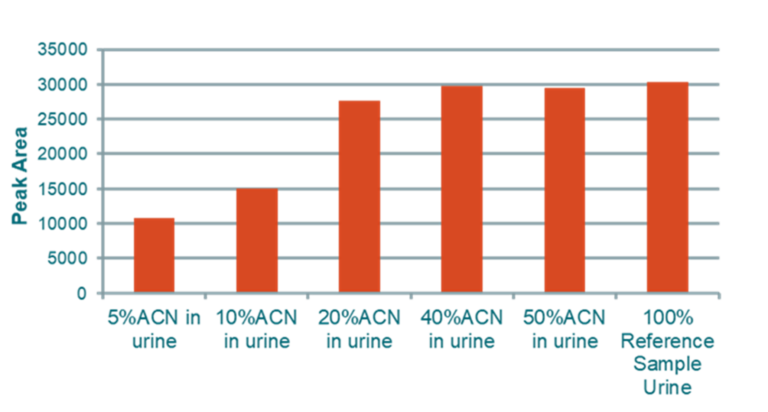
Conclusion
Utilising this generic guide as a starting point for method development of each new compound streamlined our processes and highlights issues at an early stage and creates a solid basis for future troubleshooting.