COMPREHENSIVE | PRECISE | RELIABLE
Protecting your pharmaceutical or biopharmaceutical business from foreign matter contamination is vital.
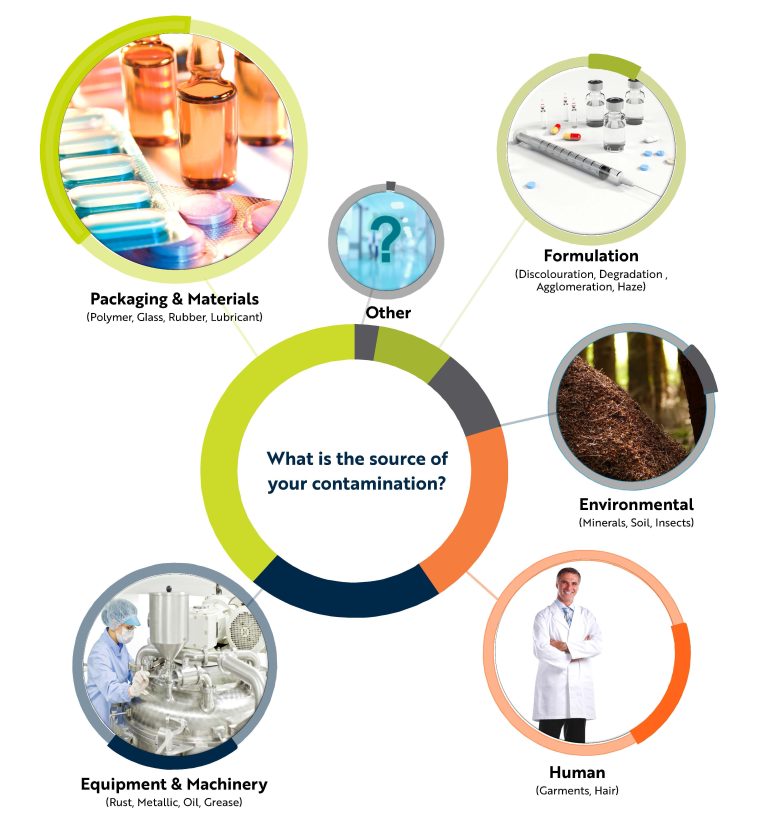
What is Foreign Particulate Matter?
Foreign matter is any material or residue that is not meant to be in the product.
The United States Pharmacopoeia Ch. 788 defines Particulate Matter (in injections) as “mobile undissolved particles, other than gas bubbles, unintentionally present in the solutions.”

The Impact of Foreign Particulate Matter?
FPM contamination can have a devastating impact on product quality, safety, and efficacy.
This can lead to costly product recalls, delays in development or production, regulatory penalties, and damage to your reputation.
Why Outsource Foreign Matter Analysis?
- Rapid response to unpredictable contamination events
- Advanced analytical tools for trace-level foreign matter analysiss.
- Data interpretation and route cause from vast experience and expertise
- A central dedicated support lab for your global manufacturing locations
Resolian contains a team of seasoned experts, with decades of experience.
- Audited by the FDA (US) and the MHRA (UK)
- Home Office controlled substance license
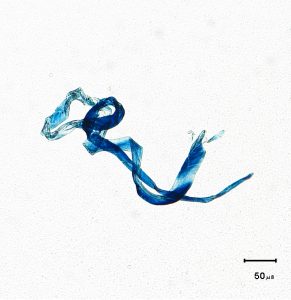
Typical Contaminates Identified in Studies Include
- Fibres
- Metallic particles
- Glass shards and delamination
- Microplastics and polymers
- Lubricant droplets
- Corrosion products
- Smears and stains
- Residues and precipitates
- Agglomeration

Your product’s integrity and safety are our top priorities.
- Protect your patients from the risks of contaminated products
- Ensure regulatory compliance
- Maintain product quality
- Achieve batch consistency
- Improve cost efficiency
- Protect your supply chain
- Support your research and development efforts