Foreign Matter Analysis
Offering a comprehensive range of testing and consulting services.
Foreign particulate matter (FPM) in vaccines poses risks to product safety, patient health, and regulatory compliance.
With stringent guidelines from agencies like the FDA and EMA, identifying and controlling contaminants is essential to maintaining trust and ensuring safety.
Where Do Contaminants Come From?
Foreign particulate matter can be introduced into your products during manufacturing, packaging, or shipping, and can come from various sources and factors, including:
- Impurities found in raw materials
- Equipment wear and tear
- Corrosion or product-packaging incompatibility
- Environmental contamination
- Cross-contamination
- Human error
- Tampering
Identification of FPM is the first step in determining its source and potential impact, which is essential for effective root cause investigations and subsequent decision-making.
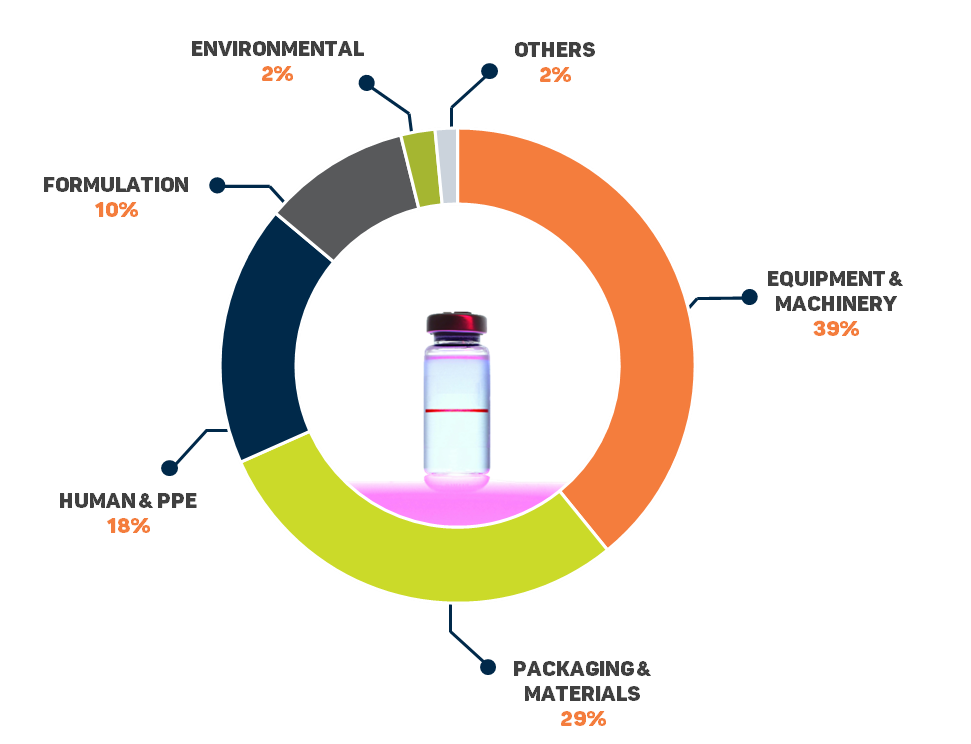
Why Outsource Foreign Matter Analysis?
It is important to have a good understanding of your manufacturing process in order to implement effective controls to prevent future contamination. However, such analyses require the right instrumentation, specialized knowledge, and the expertise in order to be useful. Therefore, it is important to have a reliable and experienced partner to help you with your investigations.
Advantages of outsourcing foreign matter analysis include:
- Access to a wide range of analytical techniques and instruments: Foreign matter analysis requires specialized equipment that is not found in every laboratory. Often, contamination occurs in trace amounts (such as a single particle), which requires specialized handling and microscopic analysis techniques.
- Access to experience and expertise: Acquiring data from the instruments is one part of the analysis; using extensive databases, expertise, and prior experience to make sense of the results is another.
- Unpredictability of occurrence: Foreign matter contamination can occur at anytime, anywhere. Therefore, dedicating and maintaining instrumentation and full-time expertise specifically for this type of analysis can be costly and resource-inefficient.
How we can help
Resolian specializes in foreign matter investigations across several industries, including pharmaceutical/biopharmaceutical, veterinary medicine, consumer healthcare, medical devices, catalysts, agrochemicals, and cosmetics.
Located in the cGMP compliant labs, Resolian provides a comprehensive range of testing and consulting services to solve your specific challenges at any stage of your product lifecycle. These services can help you to:
- Protect your patients from the risks of contaminated products
- Ensure regulatory compliance
- Maintain product quality
- Achieve batch consistency
- Improve cost efficiency
- Protect your supply chain
- Support your research and development efforts
Resolian has been audited by the FDA (US) and the MHRA (UK), and holds a controlled substance license from the Home Office, allowing for the handling and testing of all classes of controlled substances.
With a proven track record of success in helping manufacturers resolve FPM contamination issues, Resolian is committed to providing you the highest level of customer service in a timely manner.
Reach out to us directly to learn more about Resolian’s Foreign Matter Analysis services.

Typical contaminants identified in studies include:
- Fibers
- Metallic particles
- Glass shards and delamination
- Microplastics and polymers
- Lubricant droplets
- Corrosion products
- Smears and stains
- Residues and precipitates
- Agglomeration