Accelerate API Development with Resolian’s Advanced Polymorph Screening Services
Polymorph screening is a process that identifies and characterizes the crystalline forms of a compound, and then selects the best form for development.
Assessing and controlling the crystalline form of your material plays a crucial role in all the stages of the product lifecycle.
At Resolian Sandwich, we specialize in comprehensive polymorph and salt screening services to accelerate drug development across the API lifecycle. With state-of-the-art facilities and in-house capabilities, we ensure precision, efficiency, and innovative solutions at every step.
Leon (Pantelis) Xydias
How Polymorph Screening Helps
- Evaluate Drug Properties: Identify the optimal crystalline form to enhance bioavailability and performance.
- Ensure Manufacturing Control: Mitigate risks to maintain consistency and quality during production.
- Prevent Late-stage Issues: Avoid costly setbacks from unanticipated polymorph changes

Examples Where Polymorph Screening Has Proven Important
Polymorph Screening has played a vital role in these drugs:
- Chloramphenicol Palmitate (1960–1980) Polymorph screening was crucial in identifying several crystal forms of this antibiotic, helping to select the one with the best bioavailability. This process led to a stable, consistent, and more effective API formulation.
- Ritonavir Crisis (1998) During production of this HIV antiretroviral drug, polymorph screening identified a thermodynamically stable form with reduced solubility, which lowered the drug’s bioavailability. A subsequent thorough polymorph screen helped select a form with higher bioavailability, highlighting the importance of screening early to ensure stability and manufacturability in later production stages.
- Carbamazepine (1990–2000) Polymorph screening revealed multiple forms of this antiepileptic drug, each with distinct bioavailability and stability profiles. This information was key to optimizing the drug’s performance.
- Other Examples Polymorph screening has also been essential in the development of drugs like Cefdinir, Tamoxifen Citrate, Indomethacin, and Spironolactone.

Techniques Used
Powder X-Ray Diffraction (PXRD):Used to assess crystalline forms and detect subtle polymorphic differences.
Our state-of-the-art Bruker D8 Advance, with a Copper X-Ray Tube and two modes (Bragg-Brentano and Göbel mirror), is ideal for polymorph screening and routine studies. It can be equipped with an Anton Paar Temperature Controlled Sample Stage for assessing the temperature-dependent crystalline profile of API allomorphs.
Differential Scanning Calorimetry (DSC): Identifies unique melting points and thermal transitions to classify polymorphs.
Supporting techniques include Thermogravimetric Analysis (TGA), Dynamic Vapour Sorption (DVS), Hot-Stage Microscopy, and FTIR. Our new DVS systems from SMS are the industry benchmark instruments for assessing the behavior of APIs in the presence of humidity.
Resolian’s fully equipped lab in Sandwich incorporating techniques spanning Material Science, Electron Microscopy, and Analytical Chemistry, enable a thorough API study.
How Polymorph Screening Can Help Your Drug Development and the Associated Guidelines
- Early Development Stage (ICH Q6A)
- Process Development Stage (21 CFR Part 210 and 211 & ICH Q8)
- Quality Control During Manufacturing (ICH Q7 &EU GMP Annex 15)
- Formulation Development (ICH Q9)
- Post-Approval Changes and Stability Testing (ICH Q1A(R2)

Why Partner with Resolian?
- Expertise and Experience. 20+ years of polymorph screening excellence, with a global client base.
- End-to-end Study Support. Entire study conducted by Resolian, ensuring precision and efficiency from start to finish.
- Cost Efficiency. Set up, develop the methods and characterise your API in one laboratory.
- Comprehensive Lifecycle Support. Seamless integration and support from preclinical R&D to post-approval batch release.
- Scalability and Flexibility. From standalone sample analyses to high throughput testing, with bespoke packages tailored to your project needs.
- Expert Method Development and Validation. Ensure robust and reliable analytical methods that meet industry standards.
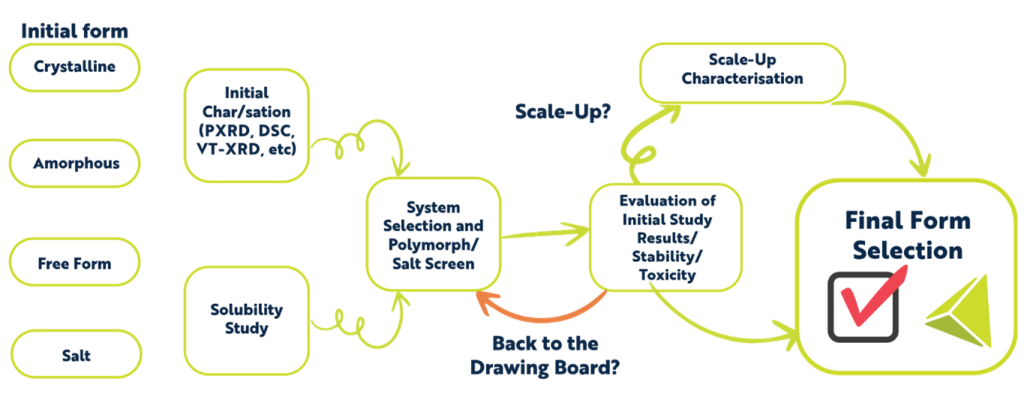
A Typical Workflow for Polymorph Screening at Resolian


