Foreign particulate matter (FPM) in vaccines poses risks to product safety, patient health, and regulatory compliance. With stringent guidelines from agencies like the FDA and EMA, identifying and controlling contaminants is essential to maintaining trust and ensuring safety.
Where Do Contaminants Come From?
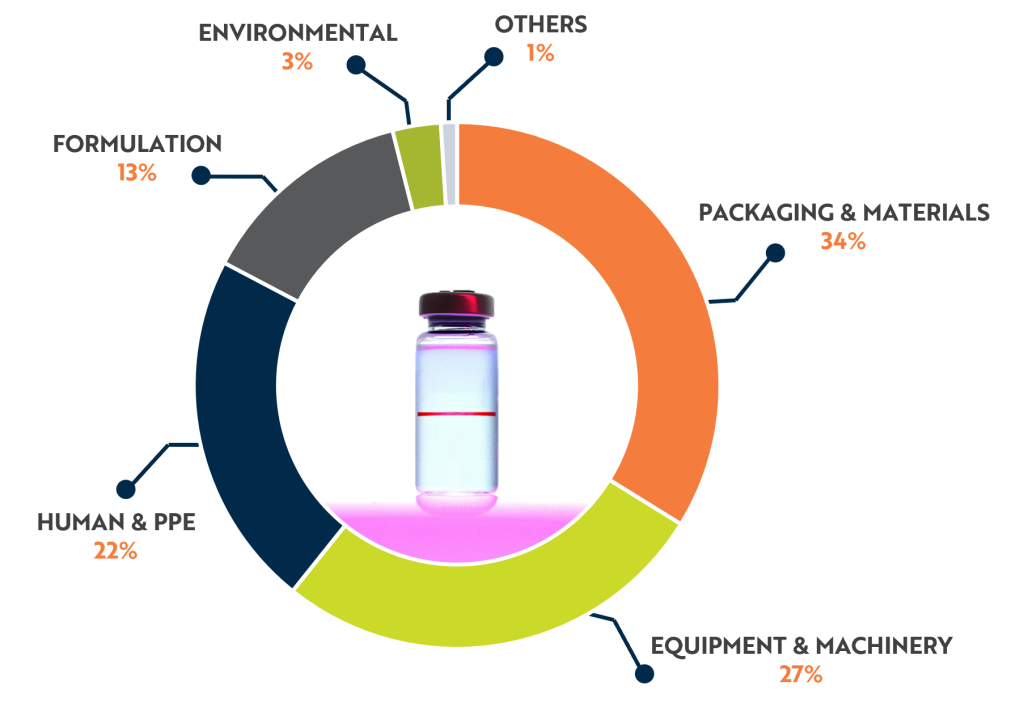
Foreign particles can enter vaccine products through various pathways:
- Raw Materials
Trace impurities in excipients or adjuvants. - Manufacturing Equipment
Wear-and-tear from machinery producing metal or rubber fragments. - Packaging
Glass shards, rubber, or silicone debris from vials and closures. - Environment
Aseptic areas and cleanrooms may introduce airborne particles. - Supply Chain
Handling and transport can compromise product integrity. - Human Interaction
Fibers or dust from clothing and gloves.
Foreign Particulate Matter Contamination Can Result In:
Adverse patient reactions such as inflammation, allergic responses, or embolism.
Costly product recalls, delays, and reputational damage.
Regulatory non-compliance, resulting in penalties or facility shutdowns
How Resolian Can Help
Resolian has a dedicated team of experts specializing in the identification of foreign particulate matter found in injectables.
Each project receives bespoke treatment paired with exceptional project management to ensure rapid response from start to finish.

Our Capabilities
- Visible and Sub-visible Particle Testing based on compendial and non-compendial methods
Contaminant isolation from syringes, cartridges, devices and other matrices
Light microscopy for information on particle size, morphology, crystallinity, and physical behaviour
FT-IR microscopy for determination of chemical composition
SEM-EDX for determination of elemental composition
PXRD for identification of crystalline particulates
Mass spectrometry (LC-MS, GC-MS) for trace impurity identification and structural elucidation
ICP-OES and ICP-MS for inorganic trace analysis
Extractables & Leachables testing
Creation of custom databases specific to your facilities to determine the source of contaminants and to identify any trends and systemic issues
Why Partner with Resolian?
- Proven Expertise:
Specialized team with decades of industry experience in contamination identification at your fingertips. - Bespoke Approach
Dedicated support tailored to your needs delivering high-quality reports backed by scientific evidence and clear conclusions. - Fast Turnaround
Rapid results to keep your operations on track.
Ensure the safety and quality of your vaccines with reliable solutions for foreign particulate matter.
Let’s work together to protect patients and your reputation. 👇

