Resolian is committed to reducing its environmental impact wherever feasibly possible.
Recently the Extractables and Leachables team at the Fordham lab of Analytical Sciences aimed to implement and evaluate green chemistry principles.
This article details the efforts and results.
About Analytical Sciences Extractable and Leachables
Extractables and Leachables (E&L) testing profiles potential impurities from primary packaging or device materials and target leachables in the final product for pharmaceutical, biopharmaceuticals, medical devices, nicotine delivery products and consumer healthcare products.
Our extensive capability in bespoke E&L assessments is in accordance with the latest regulatory guidance and utilises organic and inorganic screening methods (HS-GCMS, GCMS, LC‑HRAMS, ICP-MS) alongside diverse sample preparation techniques.

The Objective
The objective of the presented work was to implement and evaluate green chemistry principles in a liquid chromatography-high-resolution accurate mass spectrometry (LC‑HRAMS) screening method for the analysis of low volatility extractables and leachables, aiming to reduce environmental impact and improve sustainability.
The Materials and Method Solution
- The LC‑HRAMS screening method was redeveloped, acetonitrile was substituted with ethanol as organic mobile phase.
- The reversed phase method was applied with both ESI and APCI ionization techniques on a Waters Acquity I-class UPLC paired with a Synapt G2 time-of-flight mass spectrometer.
- Performance was evaluated using 21 typical leachable components purchased from Merck and Waters.
- Comparative analyses of solvent production, energy input, environmental impact, health and safety (EHS) scores, and chromatographic performance were conducted.
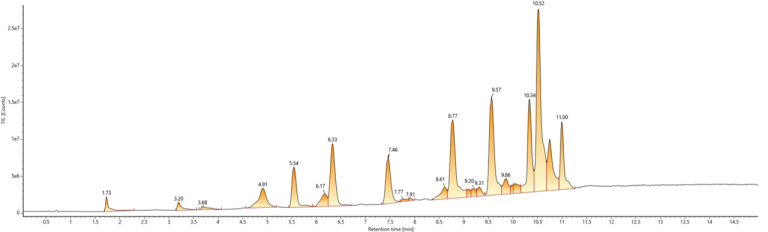
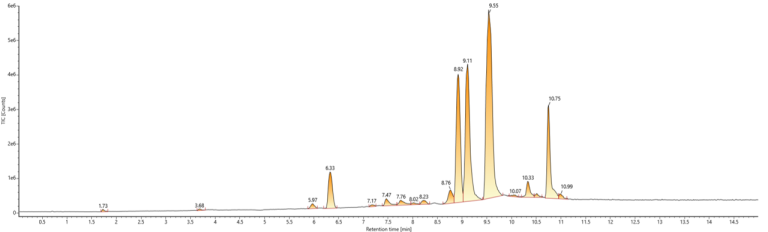
The Results
The updated method conditions led to:
- Enhanced separation of a model mixed standard
- Reduced run time: from 30 minutes to 19 minutes
- Reduced the total use of organic solvent per injection by 17%
- Decreased instrumental energy and gas consumption per injection
- Reduced laboratory running costs, due to ethanol being 42% cheaper
- Reduced exposure risk for laboratory staff
The Conclusions
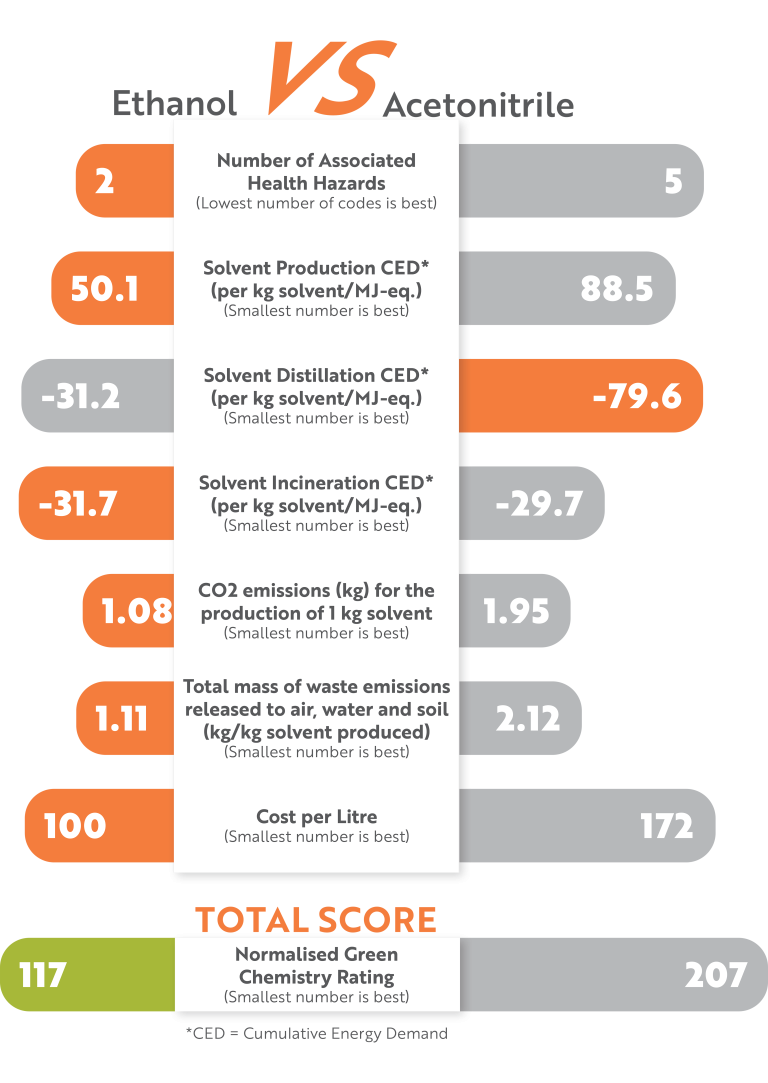
Our E&L LC‑HRAMS screening method was successfully redeveloped to use ethanol instead of acetonitrile as organic mobile phase. Analytical performance was maintained whilst gaining:
- Significant reduction in environmental impact
- Laboratory costs reduced
- The ability to incorporate bioethanol in the future
Why Resolian?
Resolian’s dedicated Extractables and Leachables (E&L) team provide the data to allow assessment of the safety and compatibility of your product with its selected storage and delivery devices, as required by regulators.
This analytical data will enable you to profile extractables from the primary packaging or device materials, and target leachables in the final product.
We will support you from early stages of development through the full lifecycle of your product.