Flow cytometric measurement of protein phosphorylation and receptor occupancy is critical for understanding a drug’s mechanism of action and binding capacity.
The Challenge
One of the main challenges of developing Flow Cytometry assays for Clinical research is whole blood sample stability. The intrinsic nature of the receptors or the target of interest (levels of expression, population frequency, target modulation, and matrix interference) add to the challenges. In many cases, stability is often less than a couple of hours and samples often must be analysed immediately, creating logistical problems.
Although several whole blood stabilization products (e.g., Cyto-Chex BCT and Transfix) extend sample stability, these have limited stability.
Correlating this data obtained from flow cytometer with other key parameters such as pharmacokinetics (PK), pharmacodynamics (PD), and immunogenicity provides deeper insight into the drug’s effectiveness on living cells.
All of which has impact on costs and quality of data.
The Solution
Our Flow Cytometry team have designed a sample processing package that solves this problem.
The process involves:
» Stabilising sample before shipping
» Batch samples from one
» Decrease variability

The Resolian Kits
Resolian provide kits containing everything the sample collection site needs:
- A,B,D are Pre-measured
- C,E,F is Pre-made from Manufacturer/Resolian
- All necessary material
- Lab Manual (The detailed Step-by-Step Lab Manual is designed to be used with entry level basic skills)
Example of RO “Split” Processing
At the sample collection site:
» Start with the Resolian Sample Processing Package Kits
» Follow the Lab Manual
» Freeze and Ship

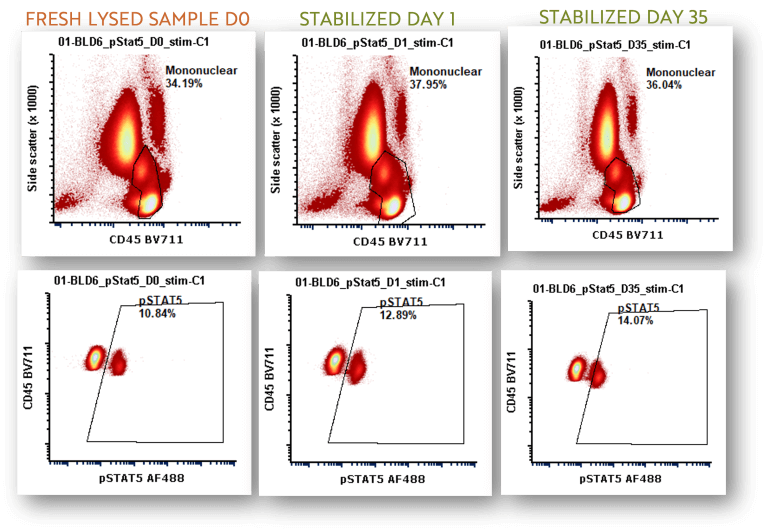
How Stable is Stable?
Freshed Lysed Blood represents traditional way of doing this. In the graph below we are showing up to 35 days post sample. The graph below shows data at 47 days with the error bars representing the replicate analysis with CV ranges between 9.4-6.2 and MdFI CV ranges between 2.0-4.7.
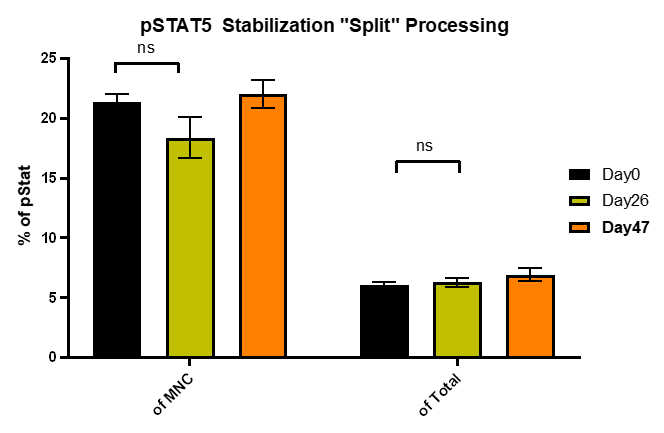
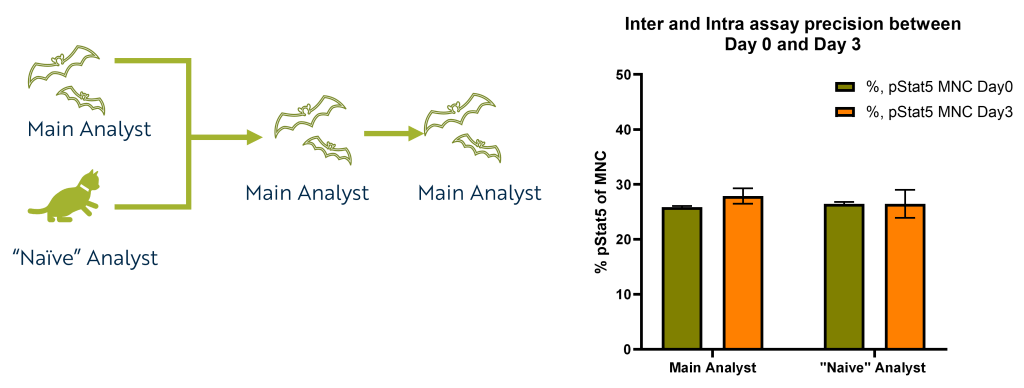
How well does it work?
The package we have designed can be used by any lab-trained staff.
For example, an analyst. Shown below is an example of a method-naive analyst generally trained in lab procedures, compared to analyst primarily working on the project.
What about other assays?
The concept of split processing works for Receptor Occupancy Assay with unique order and stabilisation:
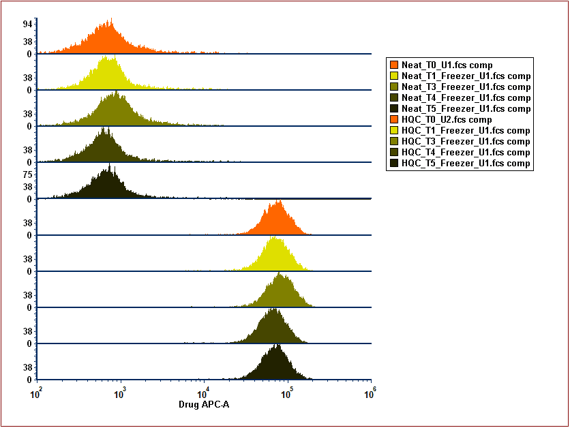
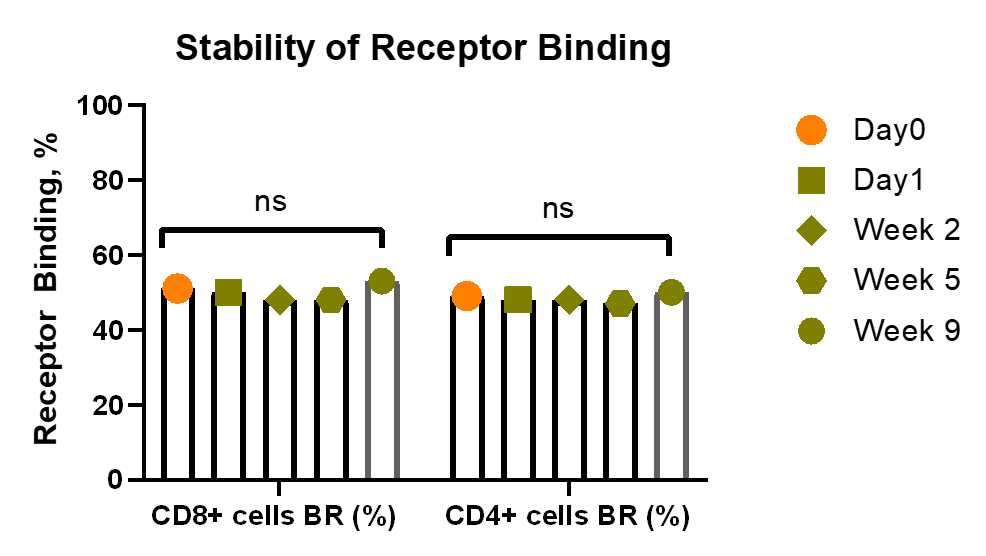

The Benefits
- Lower Batch Fees. We can now batch samples into larger batches without concerns over stability.
- Reduced Variability. Batching per subject reduces variability as the same reagent, instrument, day, and run will be used.
- Repeat Analysis. We have the luxury to repeat analysis if a batch fails.
- Automation. Batching allows us to use automation more regularly further reducing fees and improving precision.
- Multiple Centre Studies. We can handle studies conducted in multiple locations. Customs and shipping delays do not affect the samples and results.
- Low Logistics Costs. Samples can be shipped in fewer packages, reducing costs.
Working with Resolian
Our experienced team deliver seamless sample handling and management:
15mins. When the sample arrives on site it will arrive in the lab within 15 minutes.
Adaptable. Our flexible team is available to facilitate after hours and weekend sample receipt and processing.
Communication. We have built strong relationships with couriers and central lab to ensure this process works efficiently
Dedicated Resources and team
It takes a village to do Flow! Every project involves joint effort from Analysts to Scientific Director.
We pride ourselves being one big team with the right technology and people to support your project.
Learn more about Resolian’s Flow Cytometry capabilities 👉

