Resolian - Your Global CRO
Resolian is your global bioanalytical and analytical sciences partner supporting drug development, pre-clinical, and multi-regional clinical trials.
Our name is our promise
Our promise is to resolve: resolve your challenges with exceptional solutions; resolve your scientific needs through our deep knowledge and agility; resolve your projects with an open mindset and seamless communication.
Uncompromising professional and scientific integrity yields trustful, genuine relationships – and we understand that this is vital to enduring collaborations.
Our Services
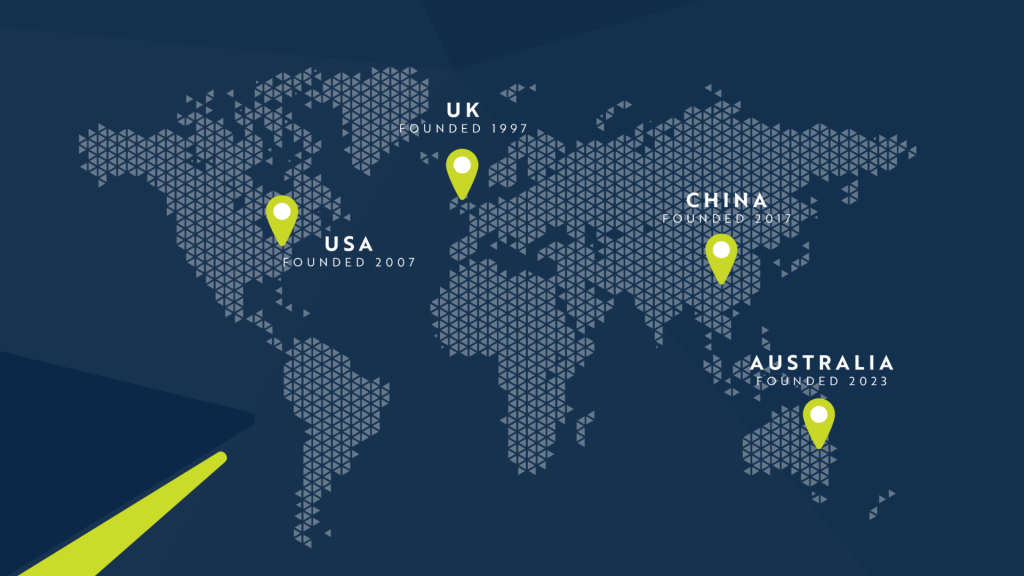
Five state-of-the-art laboratories. Four continents.
Integrated and harmonized global quality management.
Resolian boasts a global team of 30+ quality professionals proficient in GLP, GCP, cGMP, and CSV standards. With over 25 years of experience, Resolian has consistently achieved regulatory inspection success with the FDA, EPA, EMA, MHRA, and NATA, with expertise in GxP-compliant quality systems.

America


Kingdom






Choose a location
United Kingdom
Bioanalytics Fordham
Your experts in:
- LC-MS
- Immunoassay
- Biomarkers
- Protein and oligonucleotide LC-MS
- Immunogenicity
- Flow cytometry
+ Over 25 years of experience in Europe
Learn MoreAnalytical Sciences Fordham and Sandwich
Your specialist in:
- GMP and CMC testing
- Materials Science
+ Over 20 years of experience in Europe
See Fordham LabSee Sandwich Lab
North America
Bioanalytics Malvern
Your experts in:
- MetID, animal dosing (nonGLP, rodent)
- LC-MS
- Immunoassay
- Protein and oligonucleotide LC-MS
- Immunogenicity
- Biomarkers
- DMPK
- PCR
+ Over 15 years of experience in the US
Learn MoreAustralia
Bioanalytics Brisbane
Your experts in:
- LC-MS
- Immunoassay
- Biomarkers
- Early phase clinical research/phase I studies
Specialists in Australia
Learn MoreChina
Bioanalytics Chongqing
Your experts in:
- LC-MS
- Immunoassay
- Biomarkers
- Protein and oligonucleotide LC-MS
- Antibody Drug Conjugates
- mRNA assays
+ Over 20 years of industry experience
Learn MoreOur Work Culture
Join a team that thrives on innovation, collaboration, and a shared passion for excellence.